Обратная связь |
The History of Acetylene by Ralph O. Tribolet06.10.2013
История изобретения ацетилена
 Вернуться в раздел Вернуться в раздел
The History of Acetylene by Ralph O. TriboletAbout the AuthorMr. Tribolet spent forty-five years with Union Carbide Corporation’s Linde Division. Six years at Linde Engineering Laboratories specializing in acetylene production technology. Eight years in cylinder manufacturing plants as Linde resident engineer for welded, seamless, and acetylenecylinders. Ten years directing cylinder plant engineering for atmospheric gases and acetylene operations. Ten years as assistant manager of operations for Linde filling stations, including over fifty acetylene plants. Twelve years as a consultant to Compressed Gas Association for International standards and to Industrial gascompanies for cylinder plant operations and accident investigations. Mr. Tribolet holds a B.S. in Chemical engineering from Ohio State University.
Discovery of Acetylene
The name for acetylene was suggested in 1860 by Berthelot who was first to study the properties of this gas which had been discovered in 1836. But it was only after work by Moissan in 1890 had led to the manufacture of calcium carbide that acetylene assumed an industrial importance which has continued to grow.
Acetylene was first used around 1892 for lighting (especially for car headlamps). This use is now restricted to certain lighthouses and buoys. It was the invention by Charles Picard in 1901 of the oxy-acetylene blowpipe which led to the rapid development of the acetylene industry as a result of the many uses of the oxyacetylene flame in steel welding, scarfing, hard facing, surface cementation, and oxygen cutting.
Dr. Gustof Dalen of Sweden pioneered as a world-famed inventor during the early days of the acetylene industry. Dr. Dalen was the inventor of many devices using acetylene, the most important of which were those that made possible automatic acetylene lighthouses and buoys.
The marine lighting devices were used extensively throughout the world, having a flashing mechanism which conserved acetylene and a sun-valve which automatically controlled the flow of acetylene from a cylinder of dissolved acetylene. Thus, it was possible to erect lighthouses and light-buoys in desolate regions and supply them with enough fuel to last a year.
As a result of these inventions, Dr. Dalen was awarded the Nobel Prize in Physics in 1912. He was further recognized in 1932 being awarded the Morehead Medal by the International Acetylene Association (now encompassed in the CGA).
Dr. Dalen typifies the men who have been responsible for the remarkable growth of the acetylene industry. Without their inventive genius, this young industry would have never reached its present day worldwide importance in so short a time (9).
Dissolved Acetylene
"For the first few years after the commercial value of acetylene was recognized (1895) many, especially in Germany and France, attempted to handle and ship liquid acetylene, as liquid oxygen and carbon dioxide are shipped now. But many disastrous explosions resulted and the acetylene industry of today is suffering from the scare the public received during those days." (5).
Claude and Hess (38) had the idea in 1895 of using acetylene dissolved in acetone at a pressure of about 10 bars. In the following year Le Chatelier had the idea of storing this solution in containers filled with a porous mass, which had the advantage of making the gas come out in a steady stream. A small factory was opened in Malakoff near Paris in 1898 making 15m3 a day which were stored in cylinders containing acetone and a porous mass and which were intended for use in lighting trams (3).
It was soon realized that the porous mass had the advantage of increasing safety, and it is now the high quality of this mass which guarantees the safety of transport and use of cylinders of acetylene.
After 1900, the new applications for acetylene brought about the rapid development of the industry of dissolved acetylene, which offers users acetylene in a convenient form without the need to run their own generator.
Porous Mass
Porous mass needs to satisfy the following requirements:
1. Chemical stability; it must be chemically stable over perhaps decades of use, both in itself and in contact with acetylene, acetone and steel; it must not catalyze any reactions of the acetylene, acetone, or steel, alone or together.
2. High porosity; it must not detract any more than is necessary for the other requirements from the capacity of the cylinder to hold solvent and acetylene; it must not add unduly to the ratio of the total weight of the charged cylinder to the weight of the available acetylene. In many countries the porosity is limited by law to 80 per cent; in others 92 per cent is allowed.
3. Mechanical stability; in transport, use and charging, the mass must not be liable to subsidence or the production of cracks, fissures, cavities or regions of abnormally low density, whereby the efficiency of the mass from the safety point of view might deteriorate during the course of time.
4. Safety efficiency; the mass must resist the spread of acetylene decomposition, whether passed into the cylinder through equipment attached to it, or initiated at the wall of the cylinder by external heating (whether localized or general), or arising in any other way (such as heavy impact).
5. Chargeability; the mass should take up its acetylene charge in a reasonable time. This requires permeability, and the dissipation of the heat of solution of acetylene in acetone.
Dischargeability; the mass should be able to give up its acetylene readily. Discharge of gas at a rapid rate demands permeability, and the capacity to absorb the heat of solution from the surroundings.
6. Acetone retention; the mass should discharge its acetylene without entraining solvent in liquid form.
7. Cost; the acetylene cylinder is a container whose cost is ten times (or more) that of the commodity which it carries at any particular time, the porous mass should not contribute any more than is necessary to this cost differential
Many porous masses are or have been used in dissolved acetylene cylinders, and very many more have been proposed.
Fibrous Materials as Porous Mass
In the early years of the industry, cylinders in several countries were packed with kapok; many thousands of these cylinders are still in use in Britain after over thirty years of service. In the U.S.A., asbestos rope packing was used in the early years subsequently modified into asbestos discs predipped in water glass, conforming to the contour of the shell (which at that time has not neck, but a slightly concave tope welded in like the base; the preformed discs could therefore be packed into the cylinder prior to the completion of shell fabrication).
Many other fibrous materials have been proposed alone of mixed with powders, including silk, viscose, leather, sponge, flax, animal hair, glass wool, slag wool, and asbestos.
Granual Masses
Numerous materials have been proposed for the making of porous mass in granular or powder form, either alone or as mixtures sometimes with special control of grain size; they include kieselguhr, charcoal, cork, active carbon, pumice, silica gel, peat, wood or cellulose, alumina, magnesium compounds, elder pith, horn meal, cofferdam, meerschaum, sawdust, brick, etc. Kieselguhr has been proposed as bonded into granular form by melamine resins, or carboxy-methylcellulose.
Filling cylinders with these non-coherent substances is an easy matter, but most of them suffer from the disadvantage that during use they gradually settle and so no longer fill the cylinder completely, with the result that they have gradually been abandoned in favor of the coherent substances.
Monolithic Mass
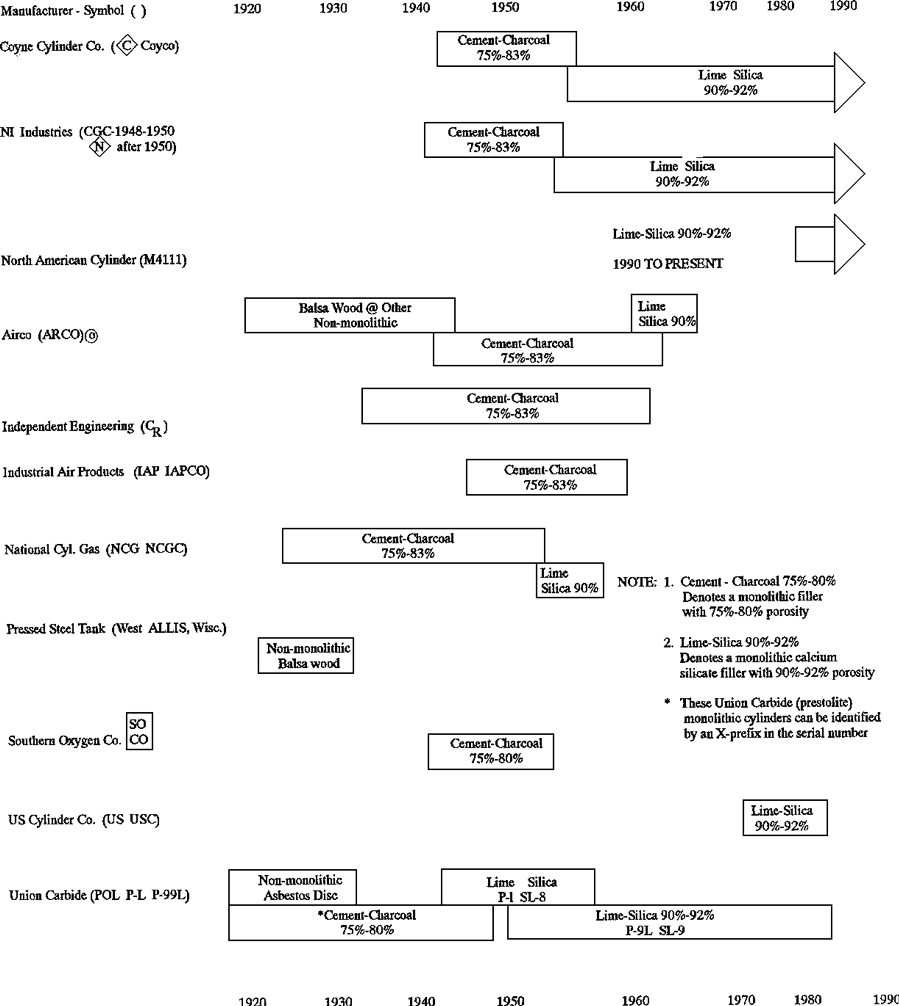
The first porous mass, made in 1897, consisted of porous ceramic bricks made by a special technique and have a porosity of 75 to 80%. Fine channels were bored into the mass to facilitate gas extraction. Because of their poor mechanical resistance these porous bricks crumbled and were soon replaced by other substances. The porous mass known in France as A.D.A.A., which is still used in a large number of cylinders, was developed in 1901. It consists of a special concrete made of pieces of charcoal and a cement made from kieselguhr, asbestos, and zinc oxychloride. This concrete with water added was put into the empty cylinder in the form of a paste and then heated to eliminate the water, forming in situ a mass which stuck to the wall and which had a porosity of between 75 and 77%. Subsequently, a porosity of 80% was achieved by using a more suitable charcoal (3).
In April 1919, the Prest-O-Lite Company obtained approval of a monolithic porous mass. All cylinders manufactured by Linde after that date contain a monolithic mass. The 1919 version was produced from charcoal, cement, kieselguhr and asbestos. These started with 70% and eventually were increased to 80% porosity.
In May 1941 the Bureau of Explosives approved a new Linde cylinder, patent No. 2,422,251, which contained a calcium-silicate monolithic mass of 81 to 83% porosity. Virtually all cylinders produced worldwide today (1992) have the calcium-silicate type porous mass with porosity as high as 92%. Regular production of the calcium-silicate mass was initiated by Linde in late 1945 at an 81/83% porosity. In 1949, Dr. McKenna of the Bureau of Explosives approved a new calcium-silicate porous mass revised for 92% porosity. Details of acetone loading, filler clearance and pore size were written into DOT 8/8AL. Patent No.
2,885,040 was issued to cover this new mass.
Cylinder Design Proof Tests
Colonel B.W. Dunn, Chief Inspector -- Bureau of Explosives to the Compressed Gass Association, September 14, 1914, wrote "For sometime I have felt that the examination, investigation and tests which this office has been able to conduct on the filling material of acetylene cylinders in order to render approval on the same under the requirements of paragraph 1961(h) of the I.C.C. Regulations have not been as complete, thought, or satisfactory as they should be." (6).
He attached thereto a proposed test regimen of 4 tests which are the basic tests required today by CGA C-12 for the design approval testing. Thus, the historical background indicates that acceptable acetylene cylinders from their inspection (1919) would pass the current day requirements.
Commercialization
Work by Moissan (France) in 1890 led to the manufacture of calcium carbide. In May 1892, J.T. Morehead, T.L. Wilson, and J.C. King were looking for a new way to refine aluminum in Spray, North Carolina. Morehead owned a cotton mill and had access to hydroelectric power. Wilson held an electric furnace patent for the production of aluminum. They wanted to adapt a process for aluminum that had been successful in steel making. It consisted of subjecting ore, together with lime and coke, to the intense heart of an electric furnace. When iron ore is used, the three materials react together. With aluminum ore, only the lime and coke reacted producing what seemed to be useless waste product.
But somehow some of the material was placed into water. It produced a pungent gas. They discovered that the material was calcium carbide, and the gas was acetylene.
While calcium carbide had been produced as early as 50 years before, the new process was the first to have large-scale commercial potential.
Four months after this accidental discovery, Wilson sent a sample of their carbide with a letter to the famous scientist, Lord William Thomas Kelvin, in Glasgow.
This established the validity of the claim those mend had made on the development of this method to produce calcium carbide. Note also that the Morehead Award for acetylene works carries the name of one of the inventors.
Interest in acetylene as a lighting source had existed in Europe for some time, and now (- 1892) that a source of acetylene was readily available, carbide production began commercially in England, Norway, Switzerland and Germany.
Shortly after the discovery in North Carolina, Morehead sold the rights to the new calcium carbide production method to financial interests in New York and Chicago, which formed the Electro-Gas Company. In 1898 the name changed to Union Carbide Company, one of five companies who form Union Carbide and Carbon Corporation in 1917. Those five companies were all involved in the production of calcium carbide, acetylene and acetylene cylinders, and oxygen.
One of the five, the Linde Air Products Company, formed in 1907, produced the first commercial oxygen in November 1907 and sold 1,545 cubic feet of oxygen. Total 1908 sales were 369,922 cubic feet which leaped to 34,600,000 cubic feet by 1912.
Also in Europe during the 1890's, scientists were busy developing another use for acetylene. It was the application of the acetylene flame to metal working. In 1895 a French chemist, Henry Louis Le Chatalier, published a paper noting that equal amounts of acetylene and oxygen produced a gas flame hotter than any flame previously known. By 1900, another Frenchman, Edmond Fouche, invented a method for controlling the oxy-acetylene flame for welding metal. Thomas Fletcher in England published some papers on flame cutting.
About this time, a meeting in a horseless carriage showroom in Indianapolis, Indiana, was to produce a very good customer for Union Carbide's calcium carbide, and change automotive lighting for years to come. The year was 1904 and those meeting were Carl Fisher and Jim Allison, proprietors of the showroom, and P.C. Avery.
The subject of the meeting was acetylene. The three felt that the automobile would have a much greater future if a practical method of lighting the car's way at night could be developed. Carbide lights were already available but required a generator to be installed on the car. Besides, it was messy, smelled, and provided only about two hours of light.
They felt the answer was to provide a way to generate the acetylene separately and pump it into a cylinder which could then be mounted on the running board of the car. There was, however, a problem because acetylene pumped into an empty cylinder was very unstable (7).
They decided to form the Concentrated Acetylene Company the same year (1904) to devise an acceptable way to store acetylene in a reasonably sized cylinder. They set up a small shop in Indianapolis. Unfortunately during the course of their efforts, the plant blew up several times. Finally, a practical, safe acetylene cylinder was developed. It consisted of a steel shell filled with a porous material which was soaked with acetone. Over the years the same porous mass has changed, but the basic concept has remained the same.
The founders wanted to expand for mass production. Fisher and Allison purchased farm land about ten miles outside Indianapolis because the city had rejected their bid to build within the city limits. The not only formed a new company but a new town became known as Speedway, the famous race town. They changed the name to the Prest-O-Lite Company, Inc. Fisher built the Indianapolis Motor Speedway in 1911.
In 1912, Prest-O-Lite obtained contracts to supply lighting cylinders for automobiles and trucks with Willys-Overland, Buick, Olds, and Reo. While Prest- O-Lite sold automotive lighting, welding was coming into its own. Meanwhile Union Carbide Company saw the potential of the new metal working method and moved rapidly to develop the vast potential market for welding. The move involved both Linde and Prest-O-Lite.
Walter Roberts was one of those responsible in Linde for developing the oxy- acetylene use. He emigrated from England to be involved in the venture to produce oxygen. He did several feats to expand the oxy-acetylene torch. One of his first jobs for Linde involved the Quebec Bridge which had fallen into the St.
Lawrence River in early 1908. Attempts to dynamite the bridge for removal were unsuccessful. Roberts convinced those in charge to let him try with an oxy- acetylene torch. He did much of the cutting himself and was, of course, successful.
An even more convincing demonstration of the cutting powers of oxy-acetylene involved dismantling boilers in the battleship Kentucky. In 1910, the ship was in Norfolk for overhaul. Workers with cold chisels and hacksaws began cutting the 1 1/8 inch boiler plate to remove the boilers. After three months they were one- quarter through the job. Roberts offered to do the job for the Navy and they were skeptical, but Roberts' claim to do it in 10 days got him the job. They set up a generator on shore and started with the torches on one end, challenging a hand crew which was no contest. The removed the boiler in less than 10 days. At the Navy's request, Linde sent two skilled torch operators to teach the Navy workmen how to use the torches (7).
Thus, our acetylene industry was born and commercialized. Starting with lighting homes and street, cooking in homes, lighting automobiles and metal workings.
The five companies who formed Union Carbide and Carbon Corporation in 1917 were: The Linde Air Products Co. (1907), Union Carbide Company ( ), Electro Metallurgical Company, National Carbon Company, Inc., and the Prest-O-Lite Company, Inc. (1912). All were involved in developing the acetylene industry in the early 1900's.
International Acetylene Association
This organization was formed in the US. in 1899 and continued as a significant factor in developing the acetylene industry. It functioned as a trade association to promulgate safety and broaden technical knowledge within the industry. In the 1960's it was melted into the Compressed Gas Association. The following is an announcement of its 37th Annual Convention as it appeared in 1936 (8).
Announcement has been made by the International Acetylene Association that it will hold its 37th Annual Convention in St Louis this fall, November 18, 19, and 20 at the Hotel Jefferson. The Association is continuing its policy of carrying its activities to many different industrial centers. In this way both the Association and the metal working industry of the city selected for the convention attain mutual benefits through a broadening of technical and engineering acquaintance and knowledge.
The registered attendance for each of the past I.A.A. conventions reflects sharply the growing interest in the oxy-acetylene process and the increasing importance attached to it by all who have industrial fabrication or maintenance problems. Over 2,300 individuals felt that the convention program last year in Cleveland contained meetings or conferences sufficiently important to warrant attendance even during the regular business hours.
Those fortunate enough to live or work in the St. Louis area by all means should plan to attend. There will be many who feel the Convention important enough to travel literally hundreds of miles as they have in the past, in order to be on hand for the valuable reports and discussions. So put in your calendar now: I.A.A. CONVENTION, HOTEL JEFFERSON, ST. LOUIS, MISSOURI, NOVEMBER 18, 19, AND 20!
The following is a list of "Miscellaneous Uses of the Oxy-Acetylene and Air- Acetylene Flames" by the Oxy-Acetylene Committee of the International Acetylene Association as published in 1945 (9). This provides some idea of the extensive efforts which expanded the acetylene industry.
The listed miscellaneous uses are: glass working, heat-treating, flame- hardening, flame-softening, carbon burning, tire mold burning, mold smoking, wood charring, heating for bending, controlled low-temperature stress relief, flame-spinning, flame-cleaning, metals coating, acetylene for paint removal, acetylene in the motion picture industry, heat-treating rail ends, low-temperature braxing, testing fire clay, splicing power belting, packing industry, baking industry, soldering, lead removal, leak detection.
Generation of Acetylene
Acetylene is generated by the reaction of calcium carbide with water. The calcium carbide used to generate acetylene is manufactured by the reduction of high quality lime by the carbon of selected cokes in the high temperature of an electric furnace. Development of the electric furnace process in the early 1900's assisted in the rapid expansion of acetylene use.
About 1930 methods based on the pyrolysis of various hydrocarbons started competing with the use of calcium carbide as a means of obtaining acetylene. These became increasingly important as a starting point for synthesizing many organic substances including, amongst others, butane diol, vinyl chloride, and other vinyl compounds (acrylonitrile, etc.). However, with the advent of ethylene chemistry, the use of acetylene as the starting stock has almost completely disappeared. In the 1950/1970 eras Union Carbide operated four large chemical complexes based on acetylene chemistry. The streams were also used for cylinder filling plants to supply the traditional acetylene industry. By the late 1980's these streams had dried-up. Data shows that acetylene production other than chemical in the U.S.A., excluding production from carbide by railroads, shipyards, welding shops and small establishments using portable generators averaged about 1.9 billion cubic feet per year for the period 1956 to 1963.
Liquid Acetylene
Acetylene can be liquefied as easily as carbon dioxide. The saturation vapor pressure at 0oC is 26.7 bars (387 psi), the liquid having a specific gravity of 0.46 g/cm3. The triple point occurs at -80.6oC and 961 mm of mercury (1.28 bars).
Thus, solid acetylene, which is a low-density substance resembling paraffin was, sublimes without melting at atmospheric pressure. Conversely, the expansion of liquid acetylene at atmospheric pressure gives a solid. When ignited in the open air, it burns quite slowly with a smoky flame.
Liquid acetylene can rather easily form in producing plants when high pressure pipelines are out-of-doors in areas where temperatures fall below 0oC. Care should be exercised to prevent such situations.
When acetylene was used for lighting, it seemed quite natural not to prepare it at the point of use but rather to store it in the liquid state in pressurized containers, as was already the practice in 1895 with other gases (SO2, NH3, etc.). The first manufacturers thought they were dealing with a liquid no more dangerous than other commonly-used liquefied gases. Thus Claude was able to raise a platinum wire to white heat in liquid acetylene at about -80oC without an explosion occurring. Consequently, in 1896 it was authorized to store liquid acetylene under pressure in metal containers which had first been subjected to a hydraulic test at 250 bars (363 psi). However, the industrial liquefaction of acetylene did not become widespread because serious accidents were reported from the outset, whilst experiments by Berthelot and Vielle showed that liquid acetylene can be easily exploded in a closed vessel at ordinary temperature, creating a pressure of more than 5 kilobars (72,500 psi). In view of its explosive power and its sensitivity to ignition, liquid acetylene has not been used commercially in the U.S.
ConclusionAn extensive industry has developed worldwide based on the use of acetylene, a truly unique gas. Its uniqueness of extreme reactivity is essential to both its beneficial use for flame temperatures or chemical synthesis. On the other hand, that feature also has limited procedures for handling and storing at pressures about 15 psig to the cylinder with porous mass and a solvent. References1. The Safe and Efficient handling of Dissolved Acetylene -- Charles Ness, Copyright 1958, Union Carbide Corp. 2. History of Acetylene Industry -- S.A. Miller, British Oxygen, Co., 1965. 3. Accidental Explosions; Volume 1; Physical and Chemical Properties -- L.A. Medard; 1st English Edition 1989. 4. "Oxy-Acetylene Tips," March 1938, Trade Journal by the Linde Air Products Co. 5. Handbook on Acetylene by Acetylene Research Department of Prest-O-Lite Company, Inc., June, 1925 6. Letter from B.W. Dunn, Chief Inspector -- Bureau of Explosives to the Compressed Gas Manufacturer's Association, Inc., Sept. 25, 1919. 7. Focus, 1982, Issue No. 2., Published by Communications Department, Linde Division. 8. Oxy-Acetylene Tips, Sept. 1936, The Linde Air Products Co. 9. Miscellaneous Uses of the Oxy-Acetylene and Air-Acetylene Flames, 1945 by the International Acetylene Association, New York, New York.
|
|||||||||||||||